Overview
The science and technology of catalysis is particularly important at this time due to the energy and environmental challenges facing society. Research in the 1990s, showing surprising activity of Au nanoparticles, has largely motivated a search for new catalytic materials on the nanoscale with properties that are different from their bulk counterparts. Another significant factor in the development of new catalysts has been the growth of computing power and improvements in theoretical methods to help understand experiments at the atomic scale and to provide guidelines for catalyst design. Experiments, demonstrating the high activity of nanoparticle catalysts, have inspired the development of theoretical methods for calculating reaction mechanisms and screening for new catalysts. Iterating between theory and experiment is a promising strategy for understanding nanoparticle catalysis and reducing the cost of the development cycle for new catalysts.
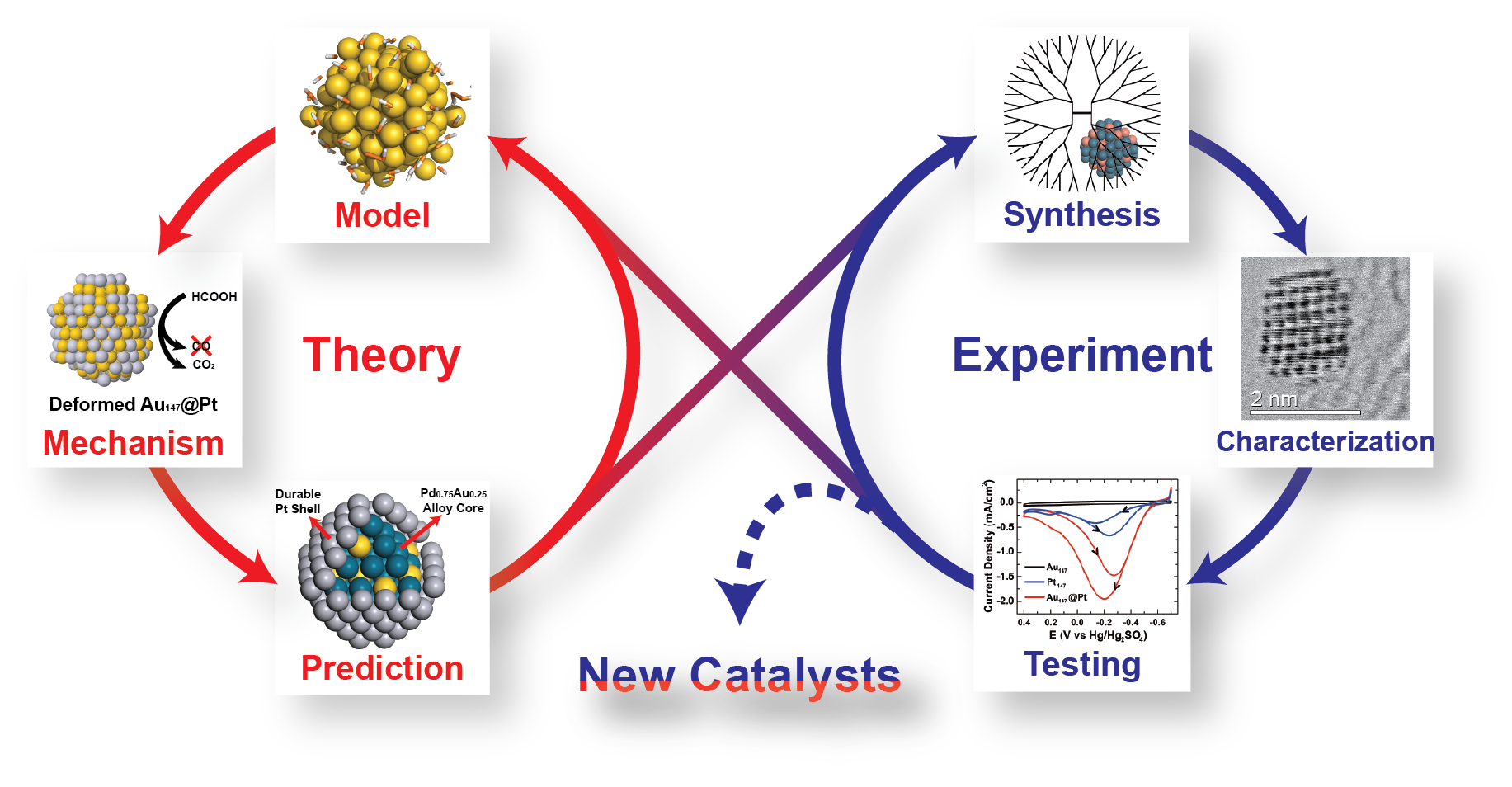
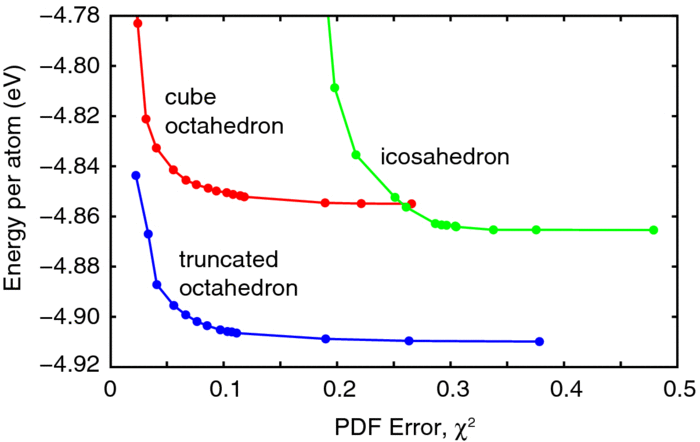
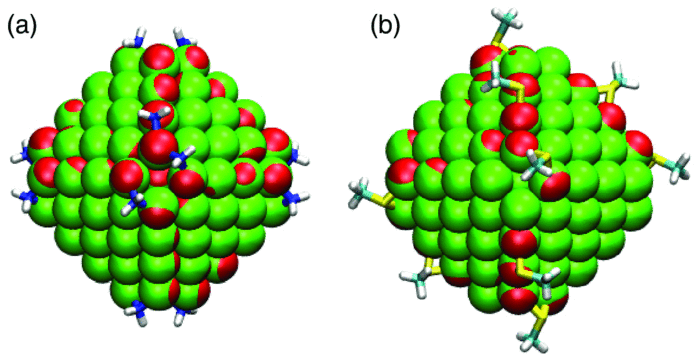
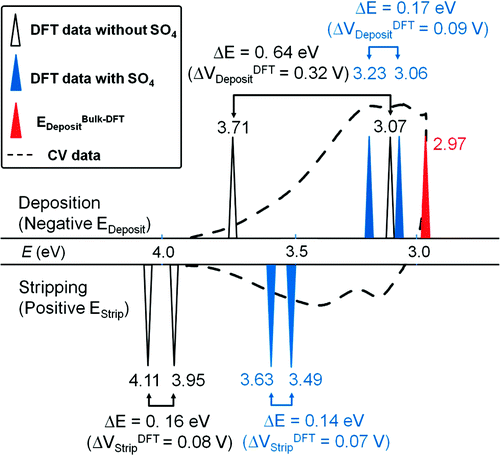
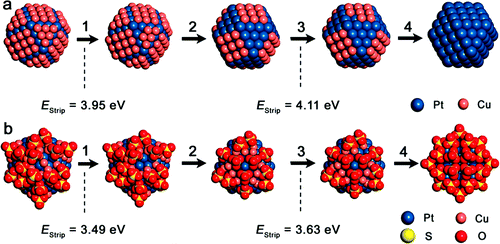
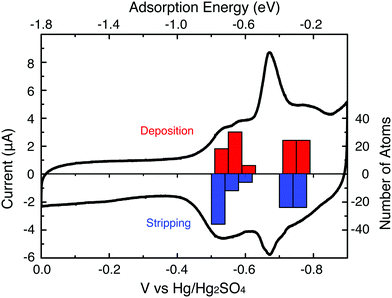
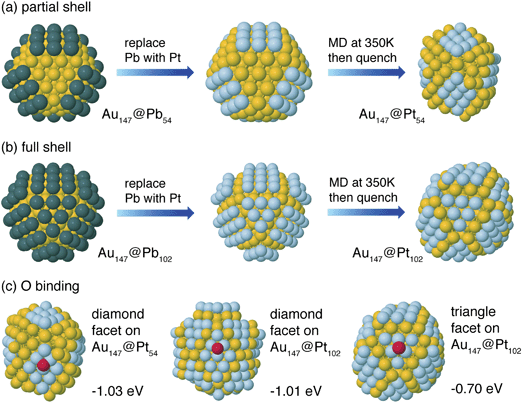
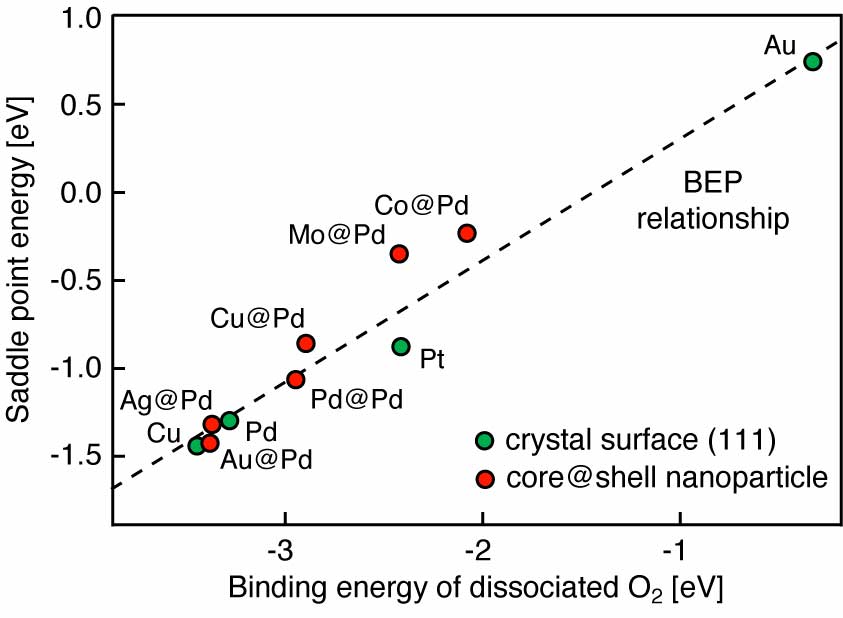
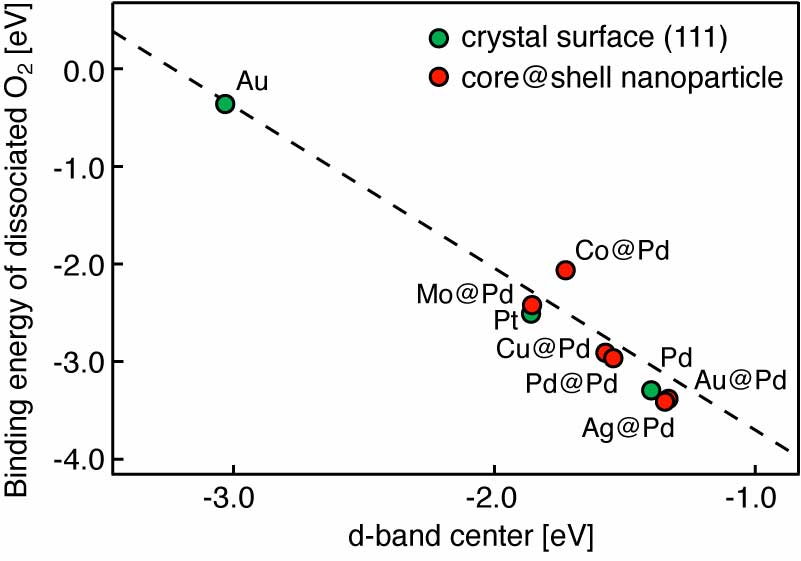
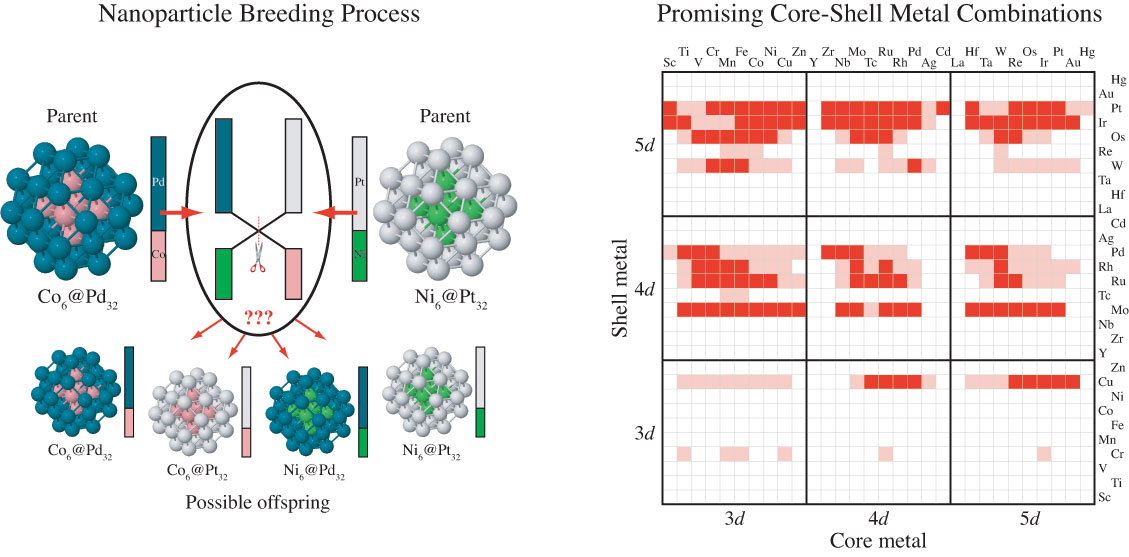
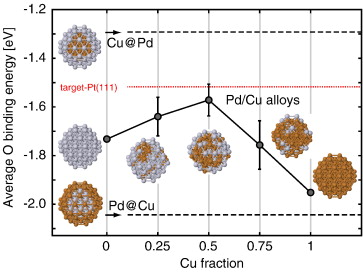
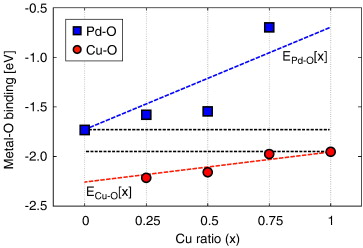
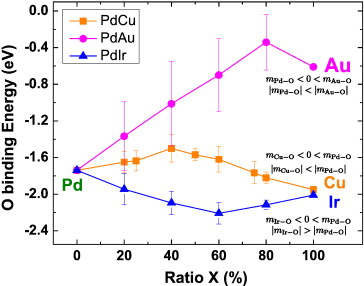
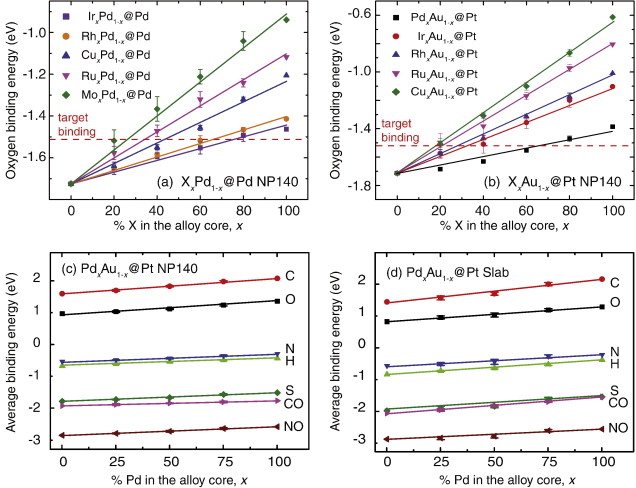
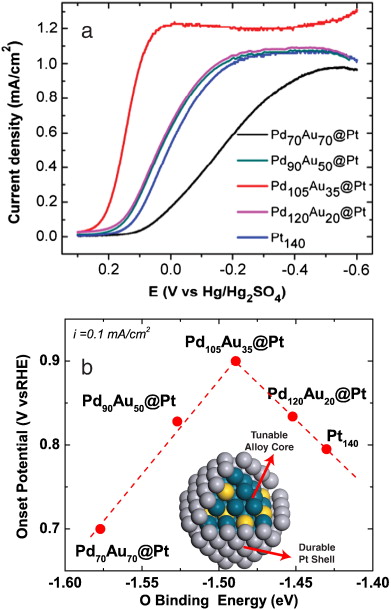
Our group tightly collaborate with experiment group lead by Prof. Richard Crooks at UT Austin, where dendrimer-encapsulated nanoparticles (DENs), as a model system are synthesized and characterized at atomic level for direct comparison with theory. Summarized by the figure above, our collaboration leads to refinement of the theory and the prediction of better nanoparticle electrocatalyst candidates. This in turn leads to the next generation of more active electrocatalysts that can be used for a wide variety of applications. Detailed review of our work has been presented in the following two papers:
1. R. M. Anderson, D. F. Yancey, L. Zhang, S. T. Chill, G. Henkelman, and R. M. Crooks, A Theoretical and Experimental Approach for Correlating Nanoparticle Structure and Electrocatalytic Activity, Acc. Chem. Res. 48 1351-1357 (2015). DOI
2. L. Zhang, R. M. Anderson, R. M. Crooks, and G. Henkelman, Correlating Structure and Function of Metal Nanoparticles for Catalysis, Surf. Sci. 640 65-72 (2015). DOI
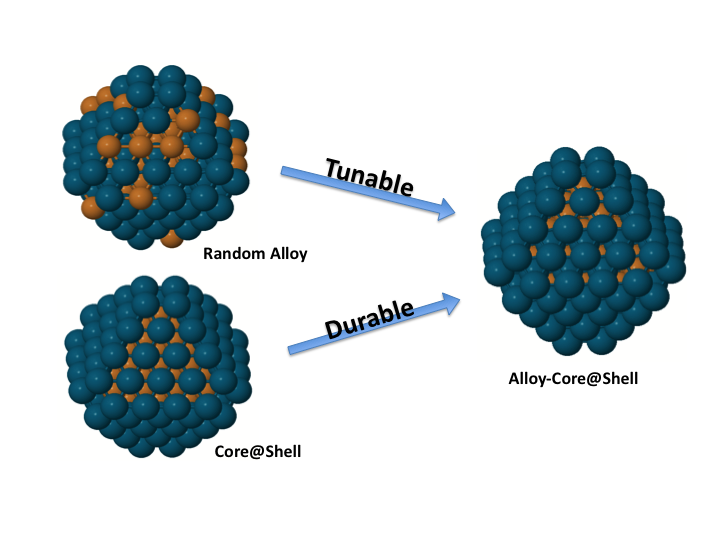
Our group also collaborates with experimental groups in UT Austin lead by Prof. Simon Humphrey and Prof. Charles Werth. Humphrey's group specializes in microwave assisted synthesis of alloy nanoparticles with conventionally immiscible metals which have catalysis applications like nitrate/nitrite reduction, methane selective activation. We use computational tools to suggest optimal alloy candidates for these catalysis applications.
Nitrate/Nitrite Reduction
Nitrate (NO3-) /Nitrite (NO2-) contamination of drinking water is a major problem in rural areas (agricultural) around the world mainly due to the extensive use of nitrogen based fertilizers. Ingestion of NO3- and NO2- leads to the formation of N-nitroso compounds which are carcinogenic, harmful for brain development of infants, causes thyroid diseases and many other health concerns. There are many ways to remove these ions like anion exchange, reverse osmosis etc but these methods have issues like generation of secondary waste, large industrial scale start-up times, high energy consumption and low ion removal efficiencies. Catalytic reduction alleviates these issues compared to the other methods, so we use direct catalytic reduction of these nitrates/nitrites to nitrogen gas (N2) which makes it easier to treat drinking water on site. One of the key challenges is to control the selectivity of the reaction products, apart from N2 gas we also get ammonia (NH3) gas as a byproduct which is not desirable because NH3 dissolves in the water making this non-potable. To control the selectivity of this reaction we used Pd-Ru (Palladium-Ruthenium) NP catalyst and found the selectivity of the reaction can be controlled by adjusting the composition of the NP. Our results show that high Pd composition favors N2 selectivity. Experimental collaborators: Dr. Werth’s and Dr. Humphrey’s groups. This project will have a potential impact on the development of cost effective ways to treat drinking water contaminated with nitrates/nitrites.